

New Solutions for Powering Medical Devices--Millimeter-scale Intraocular Pressure Sensor

2014-01-28
Non-Cytotoxic Rechargeable Batteries for Medical Devices
New Solutions for Powering Medical Devices
Small medical sensors and surgical instruments are rapidly becoming intelligent and therefore they must be powered - often using miniature rechargeable batteries. Integrated batteries must have several key properties in order to insure safe operation and protect patient health. New solid state batteries have been introduced that are uniquely fabricated using standard semiconductor manufacturing processes and packaging techniques.
In order to meet the requirements of new medical sensors and smart instruments, several factors must be considered:
- Innovative battery packaging and connectivity options must be available
- Integrated batteries must be completely non-cytotoxic
- Batteries must support heat-sterilization processes
- Various battery charging methods, including Energy Harvesting may be used
- Small size: medical sensing technologies are becoming millimeter-scale
Innovative Battery Packaging and Connectivity
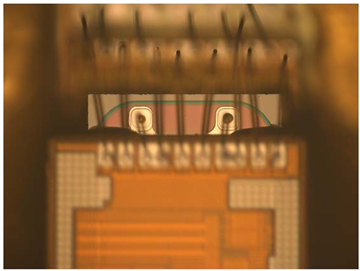
Solid state batteries have the same handling and die attach mechanisms as the other integrated circuits found in medical electronic devices. This makes solid state batteries ideal for co-packaging with other ICs to create advanced Systems-in- Package (SIP) devices. An example of the use of a solid state battery wire bond attachment in an IC stack is shown in Figure 1:
 |
|
Figure 1: Solid State Battery wire bond attachment in IC stack
|
The rechargeable solid state battery is the second layer and wire bonds to the Vout and GND pads are shown. This device stack is the actual implementation used in the Intraocular Pressure Sensor described later in this article. Solid state batteries are also available packaged in standard plastic DFN semiconductor packages that ship on tape and reel for simple, conventional surface mounting and reflow solder on a printed circuit board.
Using Solid State Batteries that are 100% Non-cytotoxic
Insuring medical product safety is absolutely critical and integrating traditional batteries into products in the past has been a problem. There are many medical applications where solid state batteries will be used either in vitro or in vivo applications. Recently, rechargeable solid state batteries have successfully passed biological safety tests in both in vitro and in vivo biocompatibility feasibility studies. During these procedures, bare die batteries were crushed and combined into a saline solution and tested in various test conditions.
In-Vitro Battery Biocompatibility Testing
The biocompatibility of the solid state batteries was evaluated using the following in vitro test methods:
Cytotoxicity: Medium Eluate Method (MEM) - 1x CMEM Cell Growth Medium Extract
Cytotoxicity: Agar Diffusion - Solid Sample
In these tests, a gamma sterilized Cymbet CBC005-BDC 5mA-hr EnerChip™ was found to be non-cytotoxic (0% cell lysis) using both the Medium Eluate Method Eluation Test and Agar Diffusion Test feasibility screening procedures. The lack of any adverse biological responses in these very sensitive in vitro cell culture assays is indicative (although not a guarantee) of biocompatible test results in the other in vitro and in vivo aspects of biocompatibility as suggested by both the EN ISO 10993-1:2009 Biological Evaluation of Medical Devices - Part 1: Evaluation and testing within a risk management process and the U.S. Food and Drug Administration (FDA) Blue Book Memorandum No. G95-1 (1995) guidelines, and is therefore another excellent reason for performing these specific and very sensitive tests.
In-Body 0% Toxicity Test Results
One of the most rigorous ways to test the intrinsic biological safety of a solid state battery is to inject crushed bare die into in vivo test settings. Crushing the battery replicates the catastrophic destruction of an EnerChip-powered implanted medical device. In this traumatic scenario, the solid state battery materials would be exposed directly to the in vivo setting. The results showed no harmful histological effects on the exposed tissues.
Meeting Additional Battery Standards and Regulations
There are also many global environmental and safety standards and directives that cover batteries. Solid state batteries are the ideal solution as they address: RoHS, China RoHS, REACH, CE Mark, UL-Underwriters Laboratory, JEDEC IC Packaging Standards, IEC, NEMA/ANSI, UN Air Safety Regulations, WEEE Directive, Battery Directive, MSDS and OSHA Information, End-of-Life Disposal Instructions and Biocompatibility Standards.
Applications in Medical Device and Food Sterilization
Process temperatures such as those reached in autoclaves used in medical device and food sterilization are generally not suitable for devices containing batteries. Temperatures reaching 137 °C are common in sterilization equipment depicted in Figure 2, and can be catastrophic to conventional batteries containing volatile solvents and other additives. Yet, there are many smart medical devices and instruments that must be processed through sterilization equipment and processes that utilize integrated batteries. Such devices include surgical tools with embedded RFID tags; implantable sensors; and temperature sensors in the equipment for enabling more precise temperature control of the equipment and contents being sterilized. Moreover, it is often essential that such sensors and RFID tags be hermetically isolated from the environment to prevent moisture ingress to the device or to prevent outgassing from the device to the environment, such as an autoclave or, in the case of implantable sensors, the human body. To insure a device is hermetic, it is very beneficial to use a hermetically sealed battery such as the Cymbet EnerChip solid state battery.
 |
|
Figure 2: Smart Medical Instrument Sterilization Equipment
|
Solving Battery Space Constraints and Footprint Requirements
Space-constrained medical devices require a small power source that occupies little volume and needs no external components (holder or socket) in order to maintain a rugged connection that will not break, separate, or corrode in harsh medical environments. Practically, apart from solid state batteries, such storage devices do not exist. Solid state batteries can be used in bare die form with solder bumps or wire bonds, or in low profile surface mount packages with or without integrated battery management, and can be recharged easily using, for example, inductive near field charging. Just as importantly, solid state batteries can tolerate the high temperatures found in autoclaves and similar equipment.
Millimeter-scale Intraocular Pressure Sensor
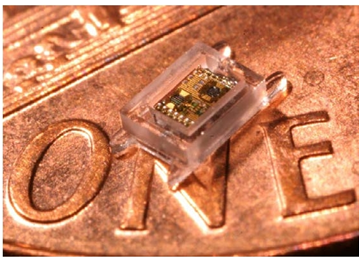
An example of a small millimeter scale Intra Ocular Pressure Sensor created to monitor the eye health of Glaucoma patients is shown in Figure 3. Several new concepts were combined to realize a tiny, intelligent sensor powered autonomously for the device life using ambient energy harvesting.
Energy harvesting techniques are used in large scale applications like solar panel installations and wind farms, but can also be used in extremely small scale devices. In this millimeter-scale example, light is converted to electricity, stored in the rechargeable solid state battery and delivered to the sensor system. There are no traditional batteries to maintain and replace, and devices can be placed anywhere.
 |
|
Figure 3: Intra Ocular Pressure Sensor (courtesy of University of Michigan)
|
A University of Michigan paper that describes the background of this device can be found here: http://www.cymbet.com/content/products-embedded-energy.asp
Using Energy Harvesting to Power the Intra Ocular Pressure Sensor
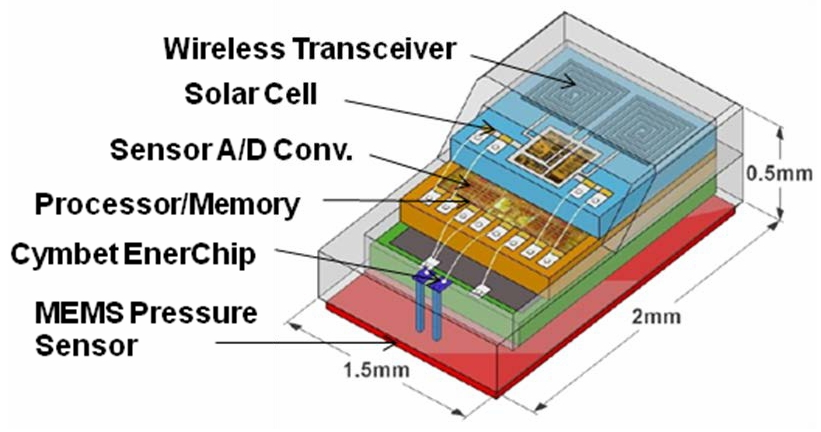
The IOP sensor shown in the Figure 3 photo is depicted diagrammatically in Figure 4. The device is a four layer stack encapsulated in a bio-compatible glass enclosure. The first layer is the MEMS pressure sensor, the second layer is a 1µAh rechargeable Cymbet EnerChip solid state battery. A processor, with memory, power management and sensor A/D converter, sits on the EnerChip as the third layer. The top layer is the solar cell and wireless transceiver. All the layers are wire-bonded together to provide electrical connectivity.
 |
|
Figure 4: IOPM Layers Block Diagram
|
Innovating New Medical Devices Using Solid State Batteries
In order to power the next generation of miniature intelligent medical sensors and instruments, solid state batteries are the correct choice. These rechargeable batteries provide the safety, size, integration, and connectivity functions required to bring successful new products to market. Moreover, all the attributes that make them ideal for medical devices can be leveraged in many other types of miniature electronic products, such as small Internet of Things environmental sensors.
There are several useful information sources available to explore all the capabilities and specifications for Rechargeable Solid State Batteries:
- The Cymbet Design Center (http://www.cymbet.com/design-center/index.php) webpage contains excellent videos, data sheets and application notes on solid state batteries
- If you would like to receive a solid state battery evaluation kit or product samples go to http://www.cymbet.com/win-a-free-enerchip-evaluation-kit.php.
- All the documents and downloads for Cymbet EnerChip information are found here: http://www.cymbet.com/products/datasheets-downloads.php.










